Comparative Oncology in VERtebrates
Multicellular organisms undergo millions to trillions of cell divisions during their lifetime, all their cells ultimately being derived from a single zygote. At every cell division, the entire genome is being copied, nucleotide by nucleotide, and although the precision of this copy mechanism is extremely high, mistakes do occur. Most of these copy errors are eliminated by very efficient and sophisticated DNA repair mechanisms that ultimately ensure almost perfect DNA replication1. Nonetheless, some errors slip through the repair machinery and become permanent mutations. Somatic mutations accumulate throughout life, particularly quickly if genes of the repair mechanisms themselves are affected by mutations, potentially resulting in the emergence of cancer. Cancer starts when a combination of somatic mutations produces cells that slip out of cell cycle control, making them able to grow and proliferate uncontrollably with rapidly accumulating genetic variation, and to invade tissues at the expense of the host organism. Cancer cells are genetically heterogeneous, often carrying different nucleotide sequences, methylation patterns of their DNA or even different chromosome numbers. These cells compete with each other for resources and space, where cells that most efficiently compete and evade programmed cell death will increase in number and frequency. Consequently, each cancer is an example of clonal evolution that is fueled by mutations and genetic diversity within the malignancy, a perfect example of a micro-evolutionary process. Hence, it is not surprising that adopting an evolutionary perspective is now regarded as key for a better understanding of oncogenic processes. Evolution not only explains how cancer emerges and progresses, but also explains why some individuals or species face lower cancer risks than others, how cancer resistance unfolded over time and predicts the age when cancer is most likely to emerge. Thus, implementing evolutionary theory in oncology promises better insight into oncogenic phenomena, potentially aiding the prevention, early detection as well as treatment of this major disease. However, despite recent progress towards greater convergence and dialogue between scientists working on oncology, ecology and evolutionary sciences, much remains to be done to achieve full integration of these disciplines. Similarly, while ecologists have ignored oncogenic phenomena, their roles in ecosystem functioning could be significant due to carcinogenesis influencing individual competitive and dispersal abilities, susceptibility to pathogens and vulnerability to predation. These processes are currently exacerbated given that most, if not all, ecosystems of our planet are now polluted with mutagenic substances.
Currently, cancer represents one of the leading causes of mortality in the human population, receiving tremendous attention from scientists, funding agencies, public and private organizations, as well as policymakers. Hence, the etiology of cancer in humans and laboratory model organisms (e.g., rats) has received ample attention, but many aspects of cancer remain poorly understood or seriously understudied. For instance, it is now widely recognized that cancer not only affects humans but also occurs in nearly every vertebrate species of the animal kingdom, from rodents to whales, in various stages from precancerous lesions to final stages, called metastatic cancers. However, despite increasing interest, our knowledge of cancer in wildlife is extremely limited, even regarding its prevalence in major vertebrate clades, its causes, consequences, life history, genetic or physiological predictors or how environmental change contributes to emerging cancer cases. Accurate estimates on cancer in wildlife promise extremely valuable information on oncogenic processes, as the limited research conducted on non-standard model organisms already provided tremendous insights into the natural mechanisms of cancer resistance. For example, very low cancer rates are ensured by duplications of the TP53 tumour suppressor gene in elephants, overproduction of high molecular mass hyaluronan in the naked mole rats, interferon-mediated concerted cell death in the blind mole rat and reduced growth hormone insulin-like growth factor-1 signalling and microRNA changes in bats. Despite its value, robust cancer prevalence data on animals was surprisingly limited until the publication of our recent paper with information for nearly 200 species of captive mammals. Our study demonstrates the universality and high frequency of oncogenic phenomena in mammals and reveals substantial differences in cancer mortality across major mammalian orders, strongly suggesting that some species are more resistant to cancer than others. Our results also highlight that cancer mortality risk is largely independent of both body mass and adult life expectancy across mammalian species. This might appear surprising since larger bodied and long-lived animals have larger cell numbers and undergo more cell divisions during their lifetime, resulting in an increased probability of accumulating somatic mutations, potentially leading to cancer. Our study thus suggests that natural selection on large size or extended longevity might have been associated with the evolution of more efficient anticancer defences. The most likely mechanism being a slower rate of mutational accumulation in large, long-lived taxa.
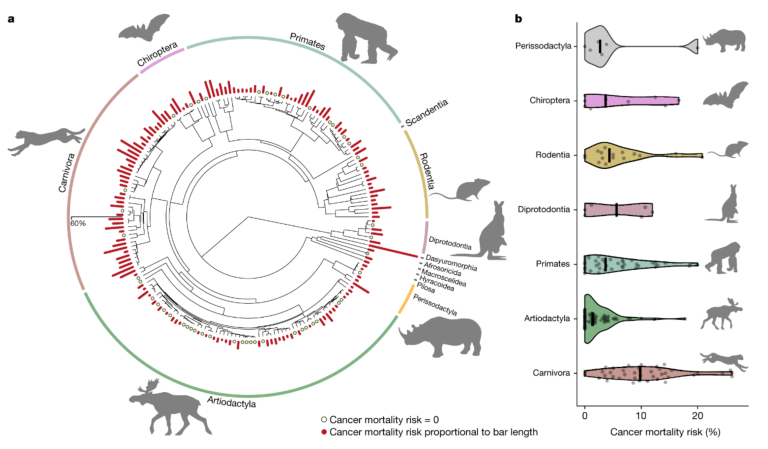
Comparative oncology made it clear that although numerous tumour suppression pathways are conserved among all multicellular organisms, some cancer resistance mechanisms are uniquely lineage-specific. Case studies are emerging rapidly and are significant contributors to a better understanding of oncogenic phenomena. Therefore, to help further advancement in this field, this project proposes to study the cross-species variance in cancer rates across vertebrates and identify key life history, physiology, genetic and cellular predictors of oncogenic phenomena. Moreover, it aims to unravel the cross-species diversity of cancer resistance and highlight future avenues in the identification of efficient tumour suppressor mechanisms. Numerous studies conducted during the past two decades have highlighted the importance of adopting an evolutionary perspective in the scrutiny of cancer. Although the insight from these studies is highly valued, the progress is slow.
This research project offers a major step forward in this field, by amassing and analyzing sorely needed comparative data on cancer prevalence, genomics and cellular adaptations across vertebrates. In the proposed research, a multidisciplinary approach will be used at the interface of oncology, physiology, genetic, cellular and evolutionary biology.
Specifically, this project proposes to describe the cross-species variability of (1) cancer prevalence, (2) genomic and (3) cellular tumour suppressor mechanisms across vertebrates.
